Copper 2 Hydroxide And Nitric Acid . to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the two ions combine together to form an insoluble salt. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. It is then converted to copper (ii). concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],.
from www.youtube.com
the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. It is then converted to copper (ii). three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the.
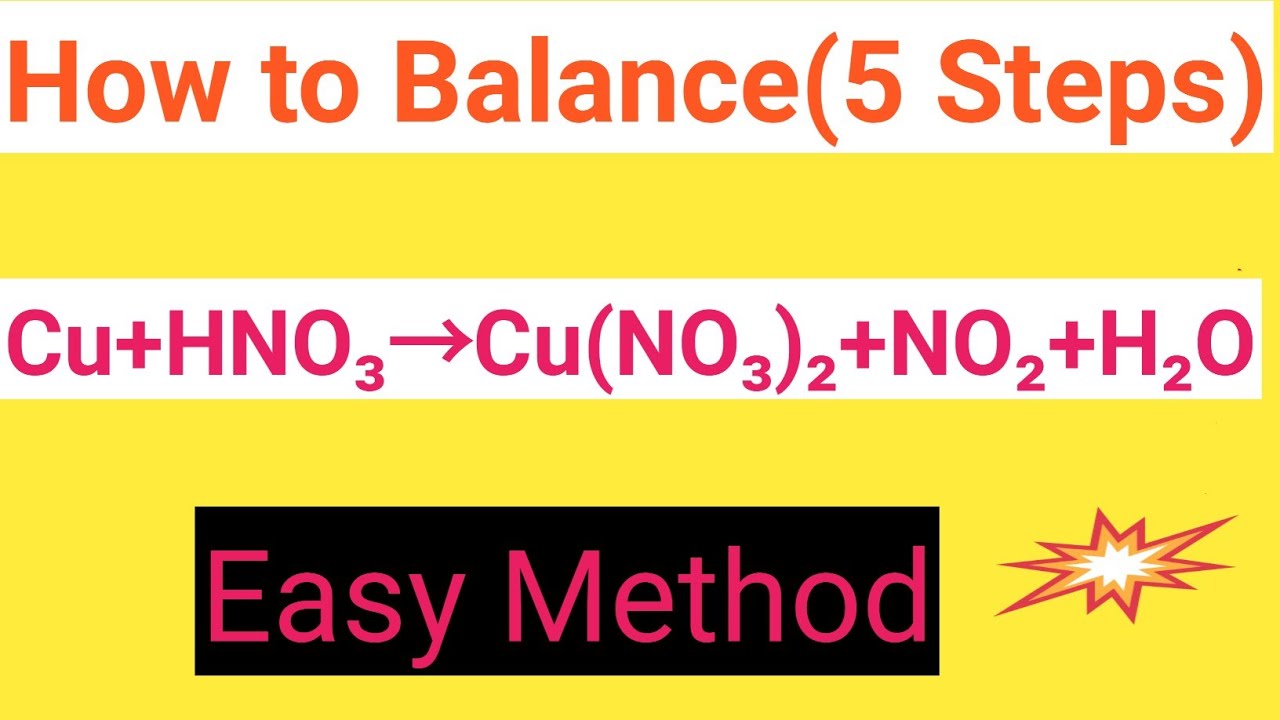
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper nitrate+Nitrogen dioxide
Copper 2 Hydroxide And Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. It is then converted to copper (ii). three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products.
From www.youtube.com
Copper and Nitric acid reaction YouTube Copper 2 Hydroxide And Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — in the first reaction, copper. Copper 2 Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546 Copper 2 Hydroxide And Nitric Acid the two ions combine together to form an insoluble salt. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. —. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation. Copper + nitric acid to cop Copper 2 Hydroxide And Nitric Acid the two ions combine together to form an insoluble salt. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. It is then converted to copper (ii). concentrated nitric acid. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper nitrate+Nitrogen dioxide Copper 2 Hydroxide And Nitric Acid — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. It is then converted to copper (ii). three moles of copper [cu] and eight moles of. Copper 2 Hydroxide And Nitric Acid.
From www.numerade.com
SOLVEDEdit question You added aqueous sodium hydroxide to the solution to neutralize excess Copper 2 Hydroxide And Nitric Acid three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper 2 Hydroxide And Nitric Acid three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. the two ions combine together to form an insoluble salt. —. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
Copper plus Nitric Acid Animation YouTube Copper 2 Hydroxide And Nitric Acid — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. It is then converted to copper (ii). to determine the products of the reaction between copper hydroxide. Copper 2 Hydroxide And Nitric Acid.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Observations... Course Hero Copper 2 Hydroxide And Nitric Acid It is then converted to copper (ii). three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. the. Copper 2 Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED Assign oxidation numbers to each of the elements in the compounds below. nitric acid Copper 2 Hydroxide And Nitric Acid three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. to determine the products of the reaction between copper hydroxide (cu (oh)2) and. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium hydroxide (NaOH)? Cu(NO3)2 Copper 2 Hydroxide And Nitric Acid It is then converted to copper (ii). the two ions combine together to form an insoluble salt. to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3). Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
Copper metal reacts with nitric acid to form a solution of copper(II) nitrate and hydrogen gas Copper 2 Hydroxide And Nitric Acid It is then converted to copper (ii). to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. concentrated nitric acid reacts with copper and produce copper. Copper 2 Hydroxide And Nitric Acid.
From brainly.in
Balance the equation Copper Hydroxide + nitric acid …… Copper nitrate + Water Brainly.in Copper 2 Hydroxide And Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate,. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Cu(NO3)2 = NaNO3 + Cu(OH)2 YouTube Copper 2 Hydroxide And Nitric Acid three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. It is then converted to copper (ii). to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. concentrated nitric acid reacts with. Copper 2 Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED Write balanced chemical equations for each reaction and describe what = in each part Copper 2 Hydroxide And Nitric Acid — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. the two ions combine together to form an insoluble salt. three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. concentrated nitric. Copper 2 Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED Question 5 (2 points) The gas produced in the reaction of the copper with nitric acid Copper 2 Hydroxide And Nitric Acid three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the two ions combine together to form an insoluble salt. — in the first reaction, copper metal. Copper 2 Hydroxide And Nitric Acid.
From brainly.in
complete the following reactions 1)reaction between nitric acid and calcium hydroxide 2 Copper 2 Hydroxide And Nitric Acid — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — in the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no 3) 2. It is then converted to copper (ii). to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid. Copper 2 Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED 1. Write the balanced equations for the following word reactions 1. carbon + oxygen Copper 2 Hydroxide And Nitric Acid concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2],. It is then converted to copper (ii). to. Copper 2 Hydroxide And Nitric Acid.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper 2 Hydroxide And Nitric Acid to determine the products of the reaction between copper hydroxide (cu (oh)2) and nitric acid (hno3), we need to consider the. concentrated nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitrogen dioxide (no 2) gas and water as products. — in the first reaction, copper metal is oxidized by nitric. Copper 2 Hydroxide And Nitric Acid.